Chemistry - Effect of periodic acid on cyclohexane derivatives
Solution 1:
I think your problem with this reaction lies in the easily confused term syn and cis. Both appear to mean that two functional groups are oriented in the same direction. On a cyclic molecule, syn and cis are the same. However, cis is a stereochemical descriptor and syn is a conformational descriptor. Simplifying this reaction to an acyclic case may help. Consider the meso isomer of 2,3-butanediol shown.
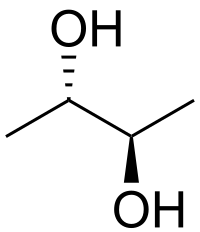
This isomer is drawn in the anti conformation, which is probably the lowest energy for steric reasons. This isomer has a syn conformation, which looks like the following. Note that the two -OH groups are not both oriented forward with solid wedges. That drawing implies a high energy eclipsed conformation.

(source: nist.gov)
Now back to the reaction with HIO4. In order to form the cyclic intermediate, the -OH groups need to be close in space. On an acyclic molecule, this requires a syn conformation, which most acyclic molecules can achieve even if it is not the lowest energy conformation. In cyclic molecules, particularly cyclohexanes, the syn conformation might not have the -OH groups close together. For cis-1,2-cyclohexanediol, which is also syn respective to the "plane" of the ring, to move the two -OH groups into the correct arrangement, it would need to wiggle into the higher energy boat or twist-boat conformations. However, trans-1,2-cyclohexanediol, which is anti has the two -OH groups oriented close together. In a sense, the trans isomer has the two -OH groups oriented syn with respect to the C-C bond that they share. Thus, trans-1,2-cyclohexanediol should react more readily with HIO4 that cis-1,2-cyclohexanediol, because the lowest energy conformer of trans-1,2-cyclohexanediol has the close arrangement of two -OH groups. The cis isomer would have to change conformation, which increases the energy input required, increasing the activation energy and slowing the reaction rate.
The diaxial case (whether boat or chair), would definitely be unreactive.
Solution 2:
The literature reveals that cis-1,2-cyclohexanediol 1 cleaves about 20-times faster than trans-1,2-cyclohexanediol 2 although the equilibrium between the diol and the cyclic periodate intermediate slightly favors the trans-isomer. The rates of cleavage of trans-1,2-cyclohexanediol 2, (-)-(R,R)-2,3-butanediol 3 and meso-2,3-butanediol 4 are comparable. [The data is from the reference cited.]


Buist, G. J., Bunton, C. A. and Miles J. H., J. Chem. Soc., 1959, 743.